Our VERVE-201 product candidate targets the ANGPTL3 gene, a key regulator of cholesterol and triglycerides in the liver. We believe that disrupting ANGPTL3 protein production may lead to reductions in LDL-C and remnant cholesterol levels through a mechanism distinct from that of PCSK9.
We are utilizing our internally developed GalNAc-LNP to deliver a base editor targeting the ANGPTL3 gene to the liver. In patients with homozygous familial hypercholesterolemia (HoFH), an orphan genetic disease characterized by extremely high LDL-C levels, delivery of base editors with standard lipid nanoparticles (LNPs) to the liver is challenging due to the deficiency of low-density lipoprotein receptor (LDLR), which is known to mediate LNP uptake. We have developed proprietary LNPs with a GalNAc ligand designed to bind to asialoglycoprotein receptors (ASGPR) in the liver, thereby enabling uptake into the liver in HoFH patients.
We plan to develop VERVE-201 to initially target two ASCVD indications with unmet medical need: HoFH and refractory hypercholesterolemia (RH). RH is defined as patients with ASCVD who are not at their medically recommended LDL-C goal despite being on maximally tolerated standard of care therapies.
We are currently conducting the Pulse-1 Phase 1b clinical trial, which is designed to evaluate the safety and tolerability of VERVE-201 in adult patients with RH.
Encouraging Nonclinical Results
In a long-term study of 34 non-human primates (NHPs), we administered a control (n = 12) or VERVE-201cyn – the NHP surrogate of VERVE-201 – at a dose of 1.5 mg/kg (n = 6) or 3.0 mg/kg (n = 16). At the higher dose, this treatment led to dose-responsive mean whole liver ANGPTL3 gene editing of 63% and mean blood ANGPTL3 protein reduction from baseline of 96% in wild-type NHPs out to six months following treatment, with durable mean blood ANGPTL3 protein reduction from baseline of 92% (n=10) out to 22 months following treatment.
In this NHP study, VERVE-201cyn was well-tolerated, with only transient elevations in liver-related biomarkers – as is typical for medicines delivered by an LNP – that fully normalized within 14 days and a reduction in liver triglycerides, a marker of liver steatosis (‘fatty liver’ disease). The reduction in ANGPTL3 protein has proven durable more than six months after dosing, supporting the potential for VERVE-201 to have a permanent treatment effect.
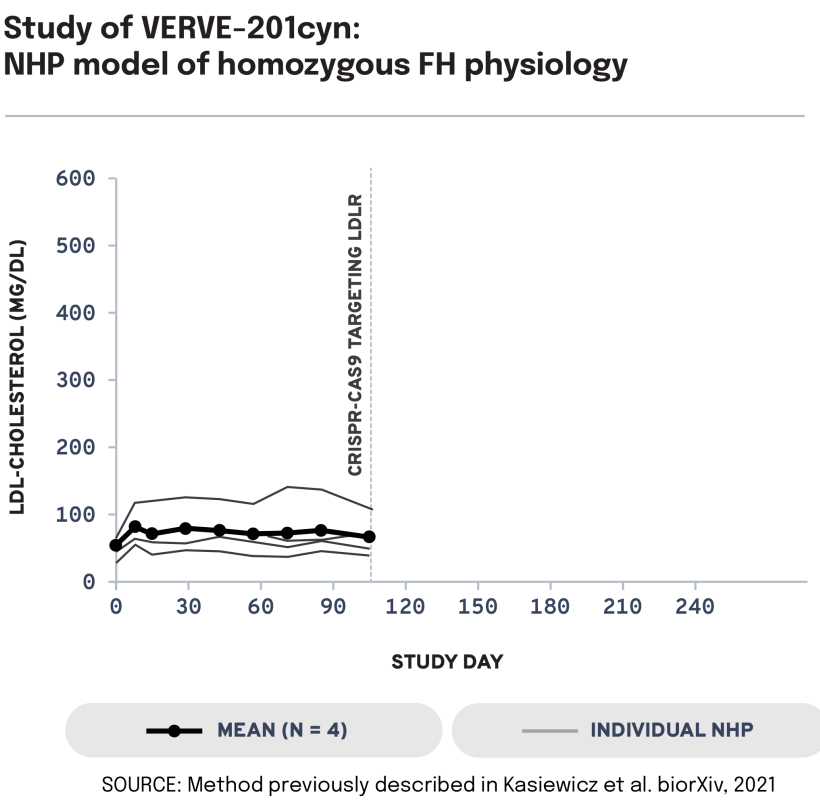
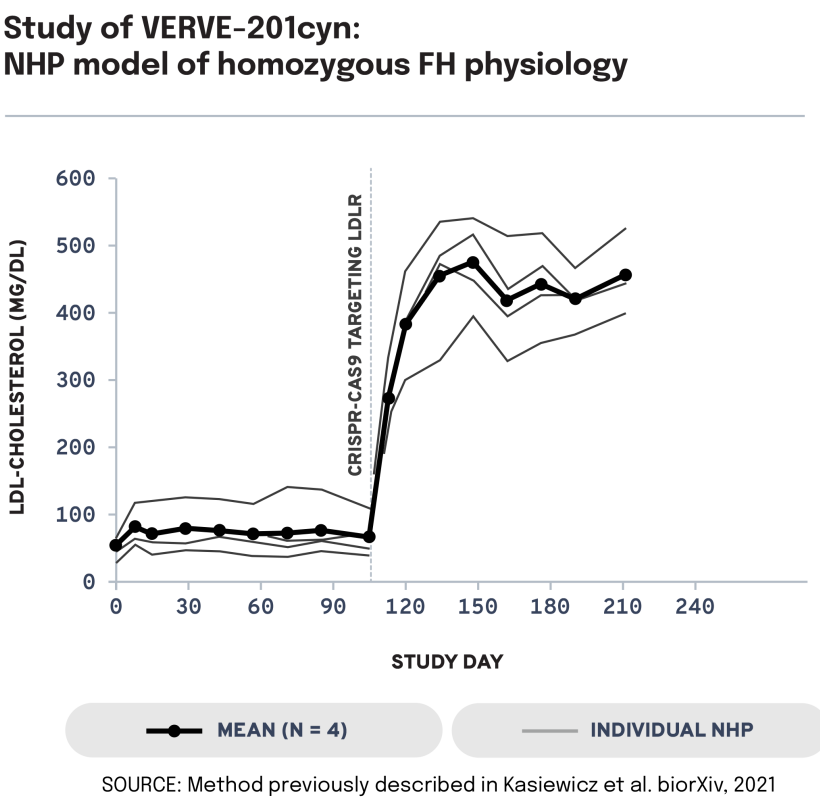
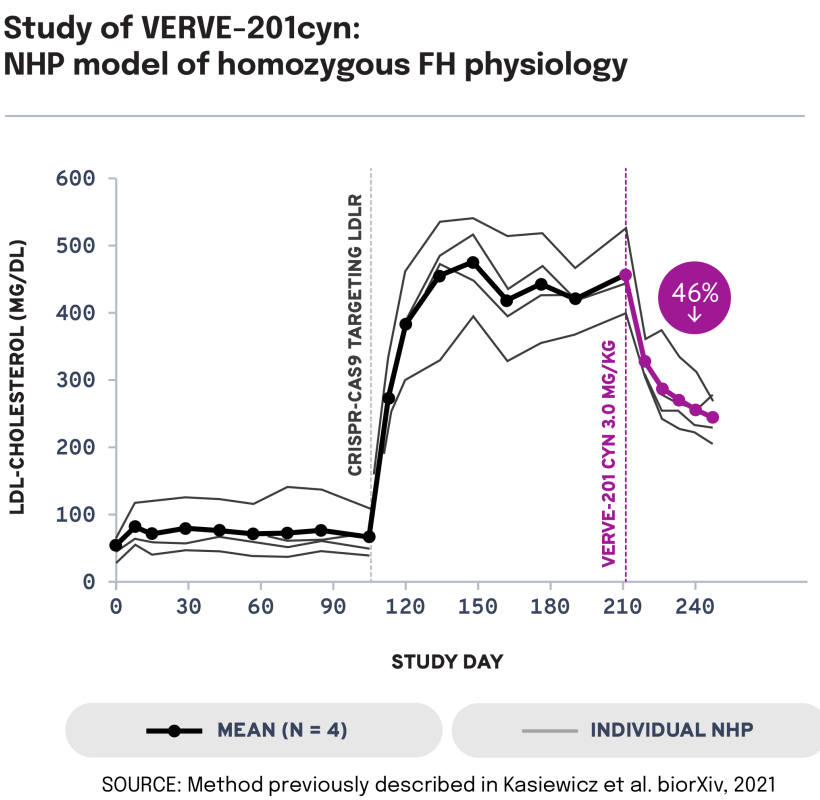
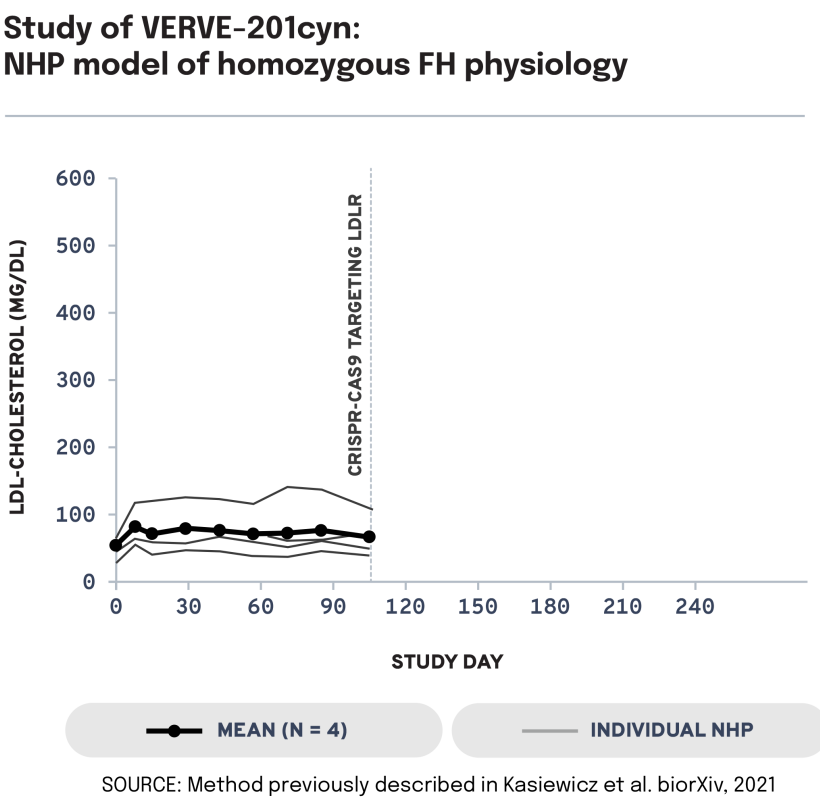
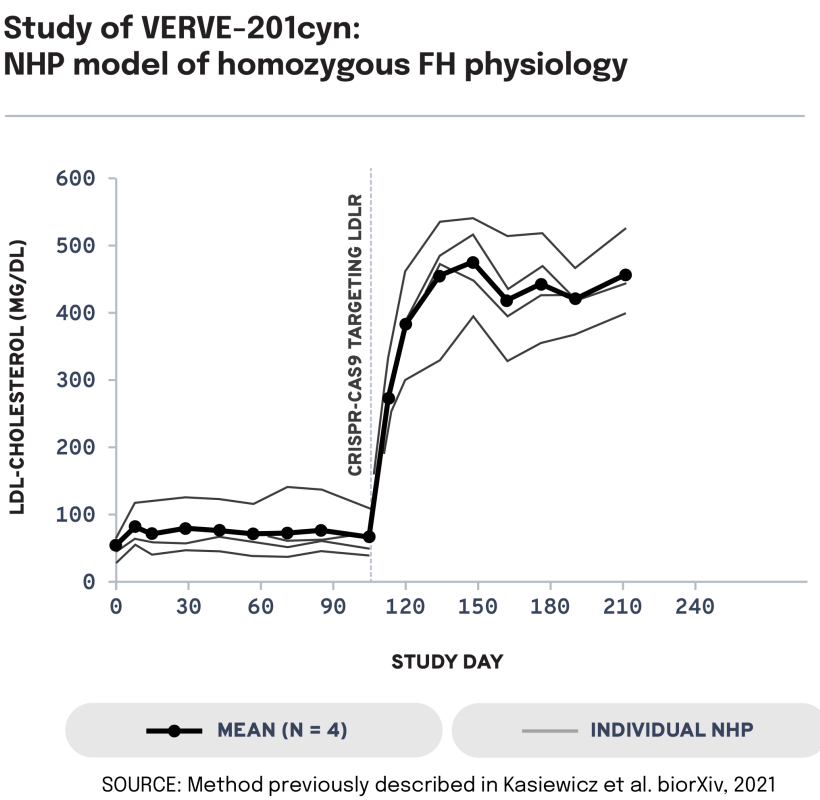
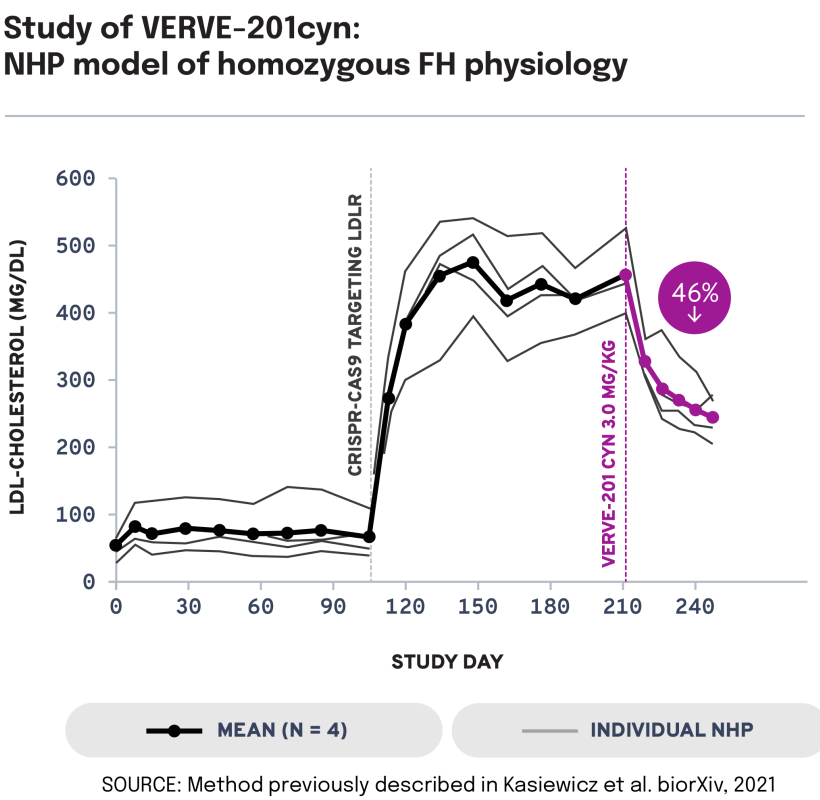
We next evaluated the potential of VERVE-201cyn to edit the ANGPTL3 gene in an NHP model of LDLR deficiency. In LDLR-deficient NHPs treated with VERVE-201cyn (n = 4), we observed 60% mean whole-liver editing and a 46% reduction in mean LDL-C from baseline of 458 mg/dL to 247 mg/dL. These data support the potential of Verve’s proprietary GalNAc-LNP – designed to enable delivery into the liver even in patients with severe or complete LDLR deficiency – to treat patients with HoFH.